Background
Double-expression lymphoma (DEL), a type of diffuse large B cell lymphoma (DLBCL) with co-expression of MYC and BCL2 proteins without gene rearrangements, is associated with poor prognosis for patients. The double expression of MYC and BCL2 may result in the activation of the B cell receptor (BCR) signaling pathway. Bruton tyrosine kinase (BTK) serves as a rational therapeutic target for DEL due to its crucial role in BCR signaling. The Phoenix study demonstrated that ibrutinib (first generation of BTK inhibitor) plus R-CHOP could improve event-free survival (EFS) and overall survival (OS) in younger patients with DEL. Orelabrutinib is a novel BTK inhibitor with high selectivity to BTK. Orelabrutinib has been shown in a preclinical study that it could preserve the NK-cell-mediated antibody-dependent cellular cytotoxicity induced by rituximab, and combination with R-CHOP (O-RCHOP) displayed higher anti-tumor activity than either alone in vivo or in vitro. This study aimed to evaluate the efficacy and safety of the O-RCHOP regimen in the treatment of naive patients with DEL DLBCL.
Methods
This was a prospective, single-arm, phase II study recruiting treatment-naive patients with DEL DLBCL. DEL was defined as≥40% positive rate for MYC and ≥50% for BCL2 by immunohistochemistry. The double-hit cases (with gene rearrangement) were excluded. All patients were treated for 6-8 cycles with 21 days per cycle. They received 150 mg of orelabrutinib once daily on days 1-14 combined with R-CHOP (Rituximab 375 mg/m 2, cyclophosphamide 750 mg/m 2, liposome doxorubicin 30 mg/m 2, vincristine 1.4 mg on day 1, and prednisone 100 mg daily on days 1-5). The primary endpoint was EFS. The response was assessed after 4 or 6 cycles using PET-CT. Adverse events (AEs) were assessed according to the Common Terminology Criteria for Adverse Events v 5.0.
Results
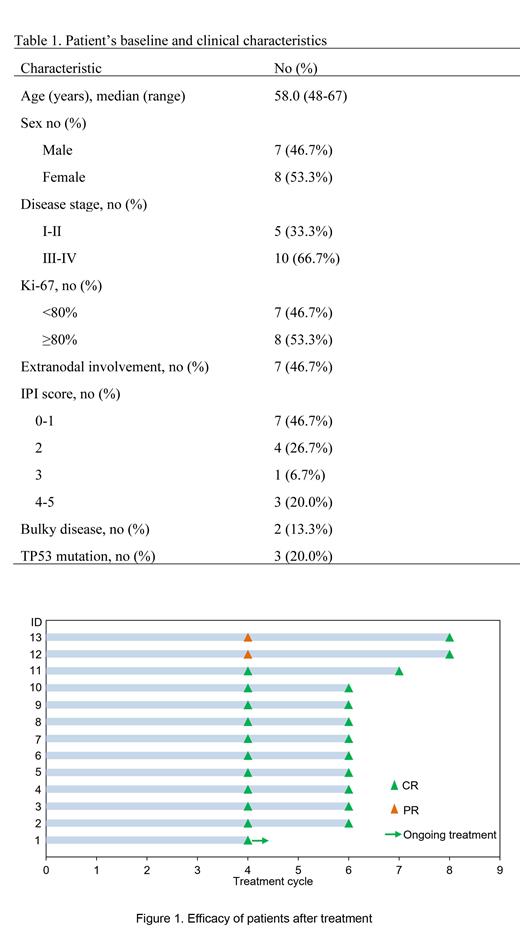
From March 27, 2021 to June 7, 2023, 15 patients with DEL were enrolled. All patients received an O-RCHOP regimen and 13 patients . The baseline characteristics of the patients were listed in Table 1. Thirteen patients who had received at least 4 cycles of treatment achieved CR (100.0%; Figure 1). After a median follow-up of 14.6 months (1.5-27.1 months), the estimated 12-month EFS rate was 100.0%. None of the 13 patients experienced disease progression or death at the cutoff date. Three of the 15 patients had the mutation of TP53 with or without other cytogenetic abnormalities tested by NGS. Grade 3/4 AEs were mainly hematological toxicity including febrile neutropenia (20%; 3/15), neutropenia (66.7%; 10/15), anemia (13.3%; 2/15), and thrombocytopenia (6.6%; 1/15), which were manageable. Neutropenia mainly occurred after the first cycle of chemotherapy, which improved after prophylaxis of PEG-rhG-CSF. The incidence of all-cause infection was 13.3% (2/15) . No atrial fibrillation, tumor lysis syndrome, or hemorrhage were observed. No patients discontinued treatment due to toxicity.
Conclusion
The combination of orelabrutinib and RCHOP showed encouraging efficacy and a tolerable safety profile in DEL DLBCL in this preliminary analysis. More updated data from this ongoing study will be provided in the future.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal